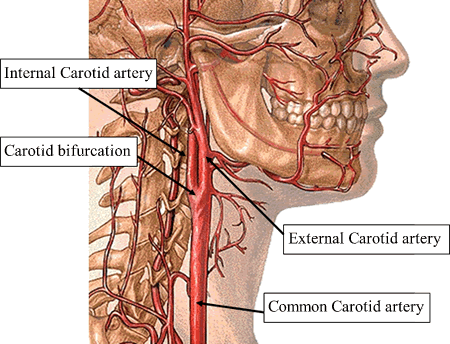
 Carotid atherosclerosis results in narrowing of the carotid arteries as plaque builds up along artery walls and at the carotid bifurcation. This narrowing (stenosis) is associated with increased risk of stroke when it progresses into the 50% to 99% range. Carotid endarterectomy (CEA) is a surgical procedure frequently used to reduce the risk of stroke by opening carotid arteries. The procedure is not without risk, however: a small but substantial minority of CEA patients suffer a perioperative stroke. This begs the question whether CEA outcomes are better or worse than medical management. For reasons explained below, this question is ripe for comparative effectiveness research.
By way of full disclosure, I am a co-investigator on an observational study of CEA vs. medical management funded by the Department of Veterans Affairs' Health Services Research & Dissemination service. My co-investigators are Steven Pizer (PI), Scott Kinlay, Graeme Fincke, Michael McWilliams, and Bruce Landon. Though based on the work of the group, what follows are my views and does not necessarily reflect the position or policy of the Department of Veterans Affairs, my co-investigators, or the other institutions with which they or I am affiliated.
As an alternative to CEA, carotid artery stenting (CAS) is a less invasive alternative that involves inserting a catheter, inflating a balloon to open the artery, and placing a stainless steel stent to keep the artery open. The Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy (SAPPHIRE) trial demonstrated that CAS is not inferior to CEA for patients with coexisting conditions that potentially increase the risk of CEA and who had either symptomatic stenosis of greater than 50% or asymptomatic stenosis greater than 80%. In this trial the probability of stroke or death from neurological causes at 1 year post randomization was not significantly different between CEA and CAS. The lack of evidence demonstrating superiority for CAS combined with a much longer history for CEA probably contribute to much larger volumes of CEA than CAS. A tabulation of the Nationwide Inpatient Sample from 2003 counted 131,565 cases of CEA and only 7,518 cases of CAS. Given the historical infrequency of CAS procedures, an observational study to compare CEA to CAS probably would be difficult.
The body of randomized trial research that demonstrates benefits and risks of CEA is also relatively old. Results from the North American Symptomatic Carotid Endarterectomy Trial (NASCET), published in 1991, demonstrated clear benefits from CEA relative to medical therapy with aspirin for patients with hemispheric or retinal transient ischemic attack or a nondisabling stroke within 120 days prior to randomization and stenosis between 70% and 99% in the symptomatic artery. The European Carotid Surgery Trial (ECST) confirmed these results, demonstrating also that the risks of CEA outweighed the benefits for those with mild stenosis (0% to 29%), and that men derived greater benefit from CEA than women. Further analysis of NASCET data indicated that symptomatic patients with stenosis between 50% and 69% also derived a moderate benefit from CEA.
The efficacy of CEA for treatment of asymptomatic high-grade carotid stenosis has been established by the Asymptomatic Carotid Atherosclerosis Study (ACAS) and the Asymptomatic Carotid Surgery Trial (ACST). The ACAS trial randomized patients with 60% to 99% stenosis to either CEA and aspirin or aspirin alone. After an average of 2.7 years of follow-up this study showed that the incidence of ipsilateral stroke and any perioperative stroke or death was significantly lower in the surgery group than the aspirin only group. However, when outcomes were limited to major ipsilateral stroke, major perioperative stroke, and perioperative death the difference between groups was no longer statistically significant. The ACST trial randomly assigned patients with greater than 60% stenosis to immediate CEA or deferral of CEA until a definite indication occurred. After an average of 3.4 years of follow-up this study found that immediate CEA reduced the 5-year risk of fatal or disabling stroke from 6.1% to 3.5%, but the immediate CEA group had a perioperative risk of stroke or death of 3.1% so they had worse event-free survival until about 2 years following surgery.
Since the ACST ceased randomization in 2003, medical management of asymptomatic carotid stenosis has changed substantially. It is, therefore, possible that aggressive medical control of hypertension, glucose, and lipids combined with antiplatelet therapy and smoking cessation might reduce the risk of stroke sufficiently to make the risks of CEA outweigh the benefits, particularly for those with less advanced stenosis. In fact, a recent review found that rates of ipsilateral and any-territory stroke, with medical intervention alone, have fallen significantly since the mid-1980s, with recent estimates overlapping those of surgical patients in randomized trials. Furthermore, rates for surgical patients from trials may underestimate actual rates because trials are conducted at high volume centers with low perioperative complication rates.
The clinical trial-based evidence on CEA was collected at a time when the comparative medical therapy consisted largely of aspirin alone. More recent advances in medical management include lipid lowering therapy, angiotensin converting enzyme (ACE) inhibitors, and clopidogrel. In clinical trials that include patients with asymptomatic carotid stenosis, medical regimens that include these newer agents reduce the risk of stroke by 50% or more, compared to older medical regimens. As a result, many investigators and clinicians are raising serious doubts about the continued efficacy of CEA for this population.
Consequently, the time is ripe for more comparative effectiveness research on CEA vs. medical management. The latter may now offer better outcomes at lower risks than prior studies could demonstrate.
Carotid atherosclerosis results in narrowing of the carotid arteries as plaque builds up along artery walls and at the carotid bifurcation. This narrowing (stenosis) is associated with increased risk of stroke when it progresses into the 50% to 99% range. Carotid endarterectomy (CEA) is a surgical procedure frequently used to reduce the risk of stroke by opening carotid arteries. The procedure is not without risk, however: a small but substantial minority of CEA patients suffer a perioperative stroke. This begs the question whether CEA outcomes are better or worse than medical management. For reasons explained below, this question is ripe for comparative effectiveness research.
By way of full disclosure, I am a co-investigator on an observational study of CEA vs. medical management funded by the Department of Veterans Affairs' Health Services Research & Dissemination service. My co-investigators are Steven Pizer (PI), Scott Kinlay, Graeme Fincke, Michael McWilliams, and Bruce Landon. Though based on the work of the group, what follows are my views and does not necessarily reflect the position or policy of the Department of Veterans Affairs, my co-investigators, or the other institutions with which they or I am affiliated.
As an alternative to CEA, carotid artery stenting (CAS) is a less invasive alternative that involves inserting a catheter, inflating a balloon to open the artery, and placing a stainless steel stent to keep the artery open. The Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy (SAPPHIRE) trial demonstrated that CAS is not inferior to CEA for patients with coexisting conditions that potentially increase the risk of CEA and who had either symptomatic stenosis of greater than 50% or asymptomatic stenosis greater than 80%. In this trial the probability of stroke or death from neurological causes at 1 year post randomization was not significantly different between CEA and CAS. The lack of evidence demonstrating superiority for CAS combined with a much longer history for CEA probably contribute to much larger volumes of CEA than CAS. A tabulation of the Nationwide Inpatient Sample from 2003 counted 131,565 cases of CEA and only 7,518 cases of CAS. Given the historical infrequency of CAS procedures, an observational study to compare CEA to CAS probably would be difficult.
The body of randomized trial research that demonstrates benefits and risks of CEA is also relatively old. Results from the North American Symptomatic Carotid Endarterectomy Trial (NASCET), published in 1991, demonstrated clear benefits from CEA relative to medical therapy with aspirin for patients with hemispheric or retinal transient ischemic attack or a nondisabling stroke within 120 days prior to randomization and stenosis between 70% and 99% in the symptomatic artery. The European Carotid Surgery Trial (ECST) confirmed these results, demonstrating also that the risks of CEA outweighed the benefits for those with mild stenosis (0% to 29%), and that men derived greater benefit from CEA than women. Further analysis of NASCET data indicated that symptomatic patients with stenosis between 50% and 69% also derived a moderate benefit from CEA.
The efficacy of CEA for treatment of asymptomatic high-grade carotid stenosis has been established by the Asymptomatic Carotid Atherosclerosis Study (ACAS) and the Asymptomatic Carotid Surgery Trial (ACST). The ACAS trial randomized patients with 60% to 99% stenosis to either CEA and aspirin or aspirin alone. After an average of 2.7 years of follow-up this study showed that the incidence of ipsilateral stroke and any perioperative stroke or death was significantly lower in the surgery group than the aspirin only group. However, when outcomes were limited to major ipsilateral stroke, major perioperative stroke, and perioperative death the difference between groups was no longer statistically significant. The ACST trial randomly assigned patients with greater than 60% stenosis to immediate CEA or deferral of CEA until a definite indication occurred. After an average of 3.4 years of follow-up this study found that immediate CEA reduced the 5-year risk of fatal or disabling stroke from 6.1% to 3.5%, but the immediate CEA group had a perioperative risk of stroke or death of 3.1% so they had worse event-free survival until about 2 years following surgery.
Since the ACST ceased randomization in 2003, medical management of asymptomatic carotid stenosis has changed substantially. It is, therefore, possible that aggressive medical control of hypertension, glucose, and lipids combined with antiplatelet therapy and smoking cessation might reduce the risk of stroke sufficiently to make the risks of CEA outweigh the benefits, particularly for those with less advanced stenosis. In fact, a recent review found that rates of ipsilateral and any-territory stroke, with medical intervention alone, have fallen significantly since the mid-1980s, with recent estimates overlapping those of surgical patients in randomized trials. Furthermore, rates for surgical patients from trials may underestimate actual rates because trials are conducted at high volume centers with low perioperative complication rates.
The clinical trial-based evidence on CEA was collected at a time when the comparative medical therapy consisted largely of aspirin alone. More recent advances in medical management include lipid lowering therapy, angiotensin converting enzyme (ACE) inhibitors, and clopidogrel. In clinical trials that include patients with asymptomatic carotid stenosis, medical regimens that include these newer agents reduce the risk of stroke by 50% or more, compared to older medical regimens. As a result, many investigators and clinicians are raising serious doubts about the continued efficacy of CEA for this population.
Consequently, the time is ripe for more comparative effectiveness research on CEA vs. medical management. The latter may now offer better outcomes at lower risks than prior studies could demonstrate.