Cardiac stents are an impressive technology. During a coronary angioplasty (aka, percutaneous coronary intervention or PCI), via a catheter snaked up from the leg, stents are inserted into coronary arteries to keep them open when they otherwise might become or remain dangerously narrow. They're an alternative to a coronary artery bypass graft (CABG), which requires cracking open the sternum. Yikes! Knowing nothing else, I'm sure you'd opt for the stent. I would too.
A lot of people do. In 2009, nearly 650,000 stent procedures were conducted, half covered by Medicare. But not all of these patients needed stents, and their overuse is hugely controversial. In a 2011 JAMA article, Paul Chan and colleagues reported that only half of all angioplasties could be verified as appropriate and 12% were found to be inappropriate (the other 38% were uncertain). The case of Dr. Mark Midei is an infamous example of overuse. As reported by Gardiner Harris in the New York Times, Midei was accused of implanting 585 unnecessary stents between 2007 and 2009, costing Medicare nearly four million dollars.
But how would one know if insertion of a stent is appropriate? Clinical trials provide useful guidance. The landmark COURAGE trial, reported in the New England Journal of Medicine (NEJM) in 2007 by William Boden and colleagues, found that as an initial therapy, stents plus medical management provided no statistically significant reduction in mortality or heart attacks compared to medical therapy alone for patients with stable angina (chest pain brought on by exercise or stress, as opposed to spontaneously while at rest). Other trials (BARI-2D, STICH) also found that outcomes for medical therapy were not improved by stents or CABG (jointly, "revascularization") for certain patients.
However, stents can relieve the symptoms of angina, increase quality of life, and reduce the need for future revascularization. (See Table 1 of the 2007 paper by Dean Kereiakes and colleagues in the Journal of the American College of Cardiology.) They may be more successful than medical therapy in doing so because adherence to pharmacological treatment is typically very low. (For example, Niteesh Choudhry and colleagues (NEJM, 2011) found that after a heart attack patients may only obtain less than half the medication they are prescribed.) Consequently, when stents are objectively determined to be appropriate, there may be reason to use them even if optimal medical therapy could achieve equivalent results on mortality and heart attack outcomes.
More recently, Bernard De Bruyne and colleagues reported on the FAME 2 trial in NEJM. This trial was similar to prior ones in that patients with stable angina were randomized to stents plus medications or medications alone. But FAME 2 added a wrinkle: patients were only randomized if they also had significant narrowing of coronary arteries as measured by fractional flow reserve (FFR, a measure of the pressure difference through the narrowing region). They found that FFR-guided angioplasty plus medical therapy decreased the need for subsequent, urgent revascularization relative to medical therapy alone. A 2010 study in Circulation by William Fearon and colleagues found that one-year costs of FFR-guided PCIs were a bit lower than non-guided ones for patients with multivessel disease and that it was cost-effective at the $50,000 per quality-adjusted life-years level.
In an editorial accompanying the FAME 2 article by De Bruyne et al., William Boden (one of the investigators on the COURAGE trial mentioned above and the ongoing ISCHEMIA trial) argued that the FAME 2 results were not strong findings since there was no difference in mortality and, because investigators were not blind to patient assignment, it is possible that they "may have had a lower threshold for recommending revascularization for a patient in the medical management group."
Nevertheless, no doubt some would argue that with FFR one can objectively identify a class of patients with stable angina for whom stents are appropriate. But let's put that debate aside for the moment. No matter how you resolve it in your own mind, it raises another issue: how do we incentivize or organize cardiologists and health systems to adhere to whatever the evidence suggests? For, no matter what, stents are not appropriate for everyone. They're a classic example of Amitabh Chandra's and Jonathan Skinner's Category II technology, which tends to be applied to a population far broader than the one for which it is most beneficial.
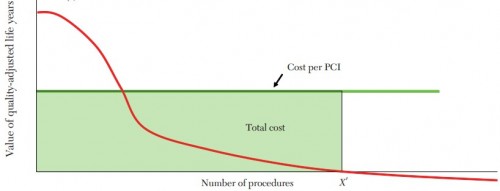
In the figure below, from Chandra and Skinner, PCI is shown to be highly beneficial for only a subset of procedures. The red line dives toward the horizontal axis and then is asymptotic to it, reflecting a large number of PCI procedures of low value. At most, FFR-guided angioplasty could somewhat reduce the number of low-value procedures, pushing the value of X' to the left. Even if that's the case, it doesn't tell us how to guide the system to achieve that reduction.
 We have not yet figured out how to prevent the cost-increasing overuse of procedures like PCI that are only beneficial to a subset of patients. Part of the problem is that health technologies are big business and providers usually get paid whether they apply the technology judiciously and appropriately or, like Dr. Midei with PCI, rampantly. PCIs, for example, are a $12 billion per year industry, or about $18,500 per procedure. Any attempt to clamp down on their application is money out of someone's pocket.
And this is the fundamental conundrum of health reform, not just the Affordable Care Act but of any type. Patients can neither determine on their own if a stent would be worth the cost nor, if insured, are they at risk for those costs. Those who are best able to make the determination, interventional cardiologists, have a financial interest, as do device manufacturers that court them. Insurers, public and private, are loath to get between the patient and provider relationship, and the law tends to keep it that way. Whatever mix of patient and provider cost sharing and/or shared decision making might steer the market to a less wasteful state and is sustainably palatable to a majority of Americans, it has not been discovered.
Existing clinical trials (or, if you're not yet convinced, future ones) may more clearly define when stents are useful and when they are not. The knowledge they provide can help define the shape of the red curve shown above. It should not be overlooked that this knowledge comes at a cost: clinical trials are not cheap. But, more importantly, that we learn their lessons is only a necessary, not sufficient condition, to actually using stents or other health technologies appropriately. As hard as it is to conduct good clinical trials, translating the results into practice is far harder. To the extent we fail to do so, we've wasted some of the cost of those trials. How do we avoid doing that?
The question of whether or not to stent is only the first of many.
Acknowledgement: Karen Joynt provided valuable feedback on an earlier draft.
We have not yet figured out how to prevent the cost-increasing overuse of procedures like PCI that are only beneficial to a subset of patients. Part of the problem is that health technologies are big business and providers usually get paid whether they apply the technology judiciously and appropriately or, like Dr. Midei with PCI, rampantly. PCIs, for example, are a $12 billion per year industry, or about $18,500 per procedure. Any attempt to clamp down on their application is money out of someone's pocket.
And this is the fundamental conundrum of health reform, not just the Affordable Care Act but of any type. Patients can neither determine on their own if a stent would be worth the cost nor, if insured, are they at risk for those costs. Those who are best able to make the determination, interventional cardiologists, have a financial interest, as do device manufacturers that court them. Insurers, public and private, are loath to get between the patient and provider relationship, and the law tends to keep it that way. Whatever mix of patient and provider cost sharing and/or shared decision making might steer the market to a less wasteful state and is sustainably palatable to a majority of Americans, it has not been discovered.
Existing clinical trials (or, if you're not yet convinced, future ones) may more clearly define when stents are useful and when they are not. The knowledge they provide can help define the shape of the red curve shown above. It should not be overlooked that this knowledge comes at a cost: clinical trials are not cheap. But, more importantly, that we learn their lessons is only a necessary, not sufficient condition, to actually using stents or other health technologies appropriately. As hard as it is to conduct good clinical trials, translating the results into practice is far harder. To the extent we fail to do so, we've wasted some of the cost of those trials. How do we avoid doing that?
The question of whether or not to stent is only the first of many.
Acknowledgement: Karen Joynt provided valuable feedback on an earlier draft.